Welcome to mathscience.in, where you’ll find comprehensive and accurate Class 9 Science Ch1 Matter in our surrounding NCERT. This chapter introduces you to the fascinating world of matter, its properties, and its various states. Whether you’re preparing for exams or looking to better understand the concepts, we have detailed solutions and explanations for every question to help you succeed.
Class 9 Science Ch1 Matter in our surrounding NCERT-Short Concept
Understanding Matter
Matter forms the basis of everything around us. It is anything that occupies space and has mass. Matter exists in three primary states: solid, liquid, and gas. Each state has distinct characteristics, and understanding these differences is crucial for mastering this chapter. Our Class 9 Science Ch1 Matter in our surrounding NCERT provide step-by-step explanations of these states, including real-life examples to help you visualize how matter behaves in various forms.
States of Matter
In NCERT Solutions for Class 9 Science Chapter 1, we explore how matter exists in three forms:
- Solids: Have a fixed shape and volume.
- Liquids: Have a fixed volume but take the shape of their container.
- Gases: Do not have a fixed shape or volume and expand to fill any available space.
Understanding the properties of each state and the differences between them is essential for grasping the key concepts of this chapter. You can find detailed explanations and examples of each state in our solutions, making it easier for you to prepare for your exams.
Changes in the State of Matter
The state of matter can change with the application of heat or pressure. Our NCERT solutions explain the processes involved in changing matter from one state to another, such as melting, freezing, condensation, and evaporation. These concepts are explained with clarity and examples to help you understand how matter behaves under different conditions.
Evaporation and Its Factors
Evaporation is an important concept covered in this chapter. It is the process by which a liquid turns into a gas. Factors such as temperature, surface area, humidity, and wind speed affect the rate of evaporation. Our solutions break down these factors and explain how they influence evaporation, providing you with the knowledge you need to excel in your understanding of matter.
Key Features of Our Class 9 Science Ch1 Matter in our surrounding NCERT
- Easy-to-understand explanations for all topics in the chapter
- Detailed answers to every question in the NCERT textbook
- Real-life examples to make complex concepts easier to grasp
- Helpful tips and tricks to remember key concepts
- Aligned with the latest CBSE syllabus, ensuring you’re fully prepared for exams
Conclusion
Mastering the concepts in Class 9 Science Ch1 Matter in our surrounding NCERT is essential for building a strong foundation in science. Our solutions are designed to make learning easy and effective, ensuring you understand the fundamental properties of matter and the changes it undergoes. Whether you’re revising for your exams or just curious about how matter works, our solutions provide the clarity you need to succeed.
Visit MathScience.in for the complete Class 9 Science Ch1 Matter in our surrounding NCERT and more resources to help you excel in your studies.
Question 1. Which of the following are matter?
Chair, air, love, smell, hate, almonds, thought, cold, cold-drink, smell of perfume.
Answer: Chair, air, almonds, and cold-drink.
Question 2. Give reasons for the following observation:
The smell of hot sizzling food reaches you several meters away, but to get the smell from cold food you have to go close.
Answer: The smell of hot sizzling food reaches severed meters away, as the particles of hot food have more kinetic energy and hence the rate of diffusion is more than the particles of cold food.
Question 3. A diver is able to cut through water in a swimming pool. Which property of matter does this observation show?
Answer: A diver is able to cut through water in a swimming pool. This shows that the particles of water have intermolecular space and has less force of attraction.
Question 4. What are the characteristics of the particles of matter?
Answer. The characteristics of the particles of matter are:
(1) Particles have intermolecular space.
(2) Particles have intermolecular force.
(3) Particles of matter are moving continuously.
Class 9 Science NCERT Textbook – Page 6
Question 1. The mass per unit volume of a substance is called density.
(density = mass/volume).
Arrange the following in order of increasing density: air, exhaust from chimneys, honey, water, chalk, cotton and iron.
Answer: Increasing density:
air < exhaust from chimneys < cotton < water < honey < chalk < iron.
Question 2. (a) Tabulate the differences in the characteristics of states of matter.
(b) Comment upon the following: rigidity, compressibility, fluidity, filling a gas container, shape, kinetic energy and density.
Class 9 Science Ch1 Matter in our surrounding NCERT-2 marks
Question 3. Give reasons
(a) A gas fills completely the vessel in which it is kept.
(b) A gas exerts pressure on the walls of the container.
(c) A wooden table should be called a solid.
(d) We can easily move our hand in air but to do the same through a solid block of wood we need a karate expert.
Answer: (a) The molecules of gas have high kinetic energy due to which they keep moving in all directions and hence fill the vessel completely in which they are kept.
(b) A gas exerts pressure on the walls of the container because the molecules of the gas are in constant random motion due to high kinetic energy. These molecules constantly vibrate, move and hit the walls of the container thereby exerting pressure on it.
(c) The molecules/particles of wooden table are tightly packed with each
other, there is no intermolecular space, it cannot be compressed, it cannot flow, all these characteristics are of solid. So wooden table should be called a solid. ‘
(d) We can easily move our hand in air but to do the same through a solid block of wood we need a karate expert. It is because the molecules of air has less force of attraction between them and a very small external force can separate them and pass through it. But in case of solids, the molecules have maximum force of attraction, the particles are tightly bound due to this force. Hence large amount of external force is required to pass through solid.
Question 4. Liquids generally have lower density as compared to solids. But you must have observed that ice floats on water. Find out why.
Answer: Ice is a solid but its density is lower than water due to its structure. The molecules in ice make a cage like structure with lot of vacant spaces, this makes ice float on water.
Class 9 Science Ch1 Matter in our surrounding NCERT Textbook – Page 9
Question 1. Convert the following temperature to Celsius scale:
(a) 300 K (b) 573 K
Answer. (a) 300 – 273 = 27°C (b) 573 – 273 = 300°C
Question.2. What is the physical state of water at:
(a) 250°C (b) 100°C
Answer: (a) 250°C = gas (b) 100°C liquid as well as gas
Question 4. Suggest a method to liquefy atmospheric gases?
Answer: The atmospheric gases are taken in a cylinder with piston fitted on it. By cooling and applying pressure on them, the gases can be liquefied.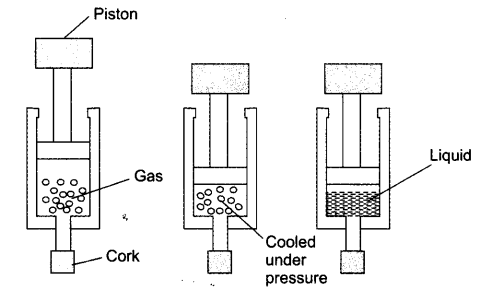
Class 9 Science Ch1 Matter in our surrounding NCERT Textbook Questions – Page 10
Question 1. Why does a desert cooler cool better on a hot dry day?
Answer: The outer walls of the cooler get sprinkled by water constantly. This water evaporates due to hot dry weather. Evaporation causes cooling of inside air of cooler. This cool air is sent in the room by the fan.
Question 2. How does the water kept in an earthen pot (matka) become cool during summer?
Answer: The earthen pot is porous with lot of pores on it, the water oozes out through these pores and the water gets evaporated at the surface of the pot thereby causing cooling effect. This makes the pot cold and the water inside the pot cools by this process.
Question 3. Why does our palm feel cold when we put some acetone or petrol or perfume on it?
Answer: Acetone, petrol or perfume evaporate when they come into contact with air. The evaporation causes cooling sensation in our hands.
Question 4. Why are we able to sip hot tea or milk faster from a saucer rather than a cup?
Answer: Tea in a saucer has larger surface area than in a cup. The rate of evaporation is faster with increased surface area. The cooling of tea in saucer takes place sooner than in a cup. Hence we are able to sip hot tea or milk faster from a saucer rather than a cup.
Question 5. What type of clothes should we wear in summer?
Answe: We should wear light coloured cotton clothes in summer. Light colour because it reflects heat. Cotton clothes because it has pores in it, which absorbs sweat and allows the sweat to evaporate faster thereby giving cooling effect.
Questions From NCERT Textbook for Class 9 Science
Question 1. Convert the following temperatures to the Celsius scale.
(a) 293 K (b) 470 K.
Answer: (a) 293 K into °C
293 – 273 = 20°C
(b) 470 K into °C 470 – 273 = 197°C
Question 2. Convert the following temperatures to the Kelvin scale.
(a) 25°C (b) 373°C.
Answer: (a) 25°C into K
25 + 273 = 298 K
(b) 373°C into K 4 373 + 273 = 646 K
Class 9 Science Ch1 Matter in our surrounding NCERT
Question 3. Give reason for the following observations.
(a) Naphthalene balls disappear with time without leaving any solid.
(b) We can get the smell of perfume sitting several metres away.
Answer: (a) Naphthalene balls disappear with time without leaving any solid, because naphthalene balls sublime and directly changes into vapour state without leaving any solid.
(b) We can get the smell of perfume sitting several meters away because perfume contain volatile solvent and diffuse faster and can reach people sitting several meters away.
Question 4. Arrange the following substances in increasing order of forces of attraction between the particles—water, sugar, oxygen.
Answer: Oxygen —> water —> sugar.
Question 5. What is the physical state of water at—
(a) 25°C (b) 0°C (c) 100°C
Answer: (a) 25°C is liquid (b) 0°C is solid or liquid
(c) 100°C is liquid and gas
Question 6. Give two reasons to justify
(a) water at room temperature is a liquid.
(b) an iron almirah is a solid at room temperature.
Answer: (a) Water at room temperature is a liquid because its freezing point is 0°C and boiling point is 100°C.
(b) An iron almirah is a solid at room temperature because melting point of iron is higher than room temperature.
Question 7. Why is ice at 273 K more effective in cooling than water at the same temperature?
Answer: Ice at 273 K will absorb heat energy or latent heat from the medium to overcome the fusion to become water. Hence the cooling effect of ice is more than the water at same temperature because water does not absorb this extra heat from the medium.
Question 8. What produces more severe bums, boiling water or steam?
Answer: Steam at 100°C will produce more severe bums as extra heat is hidden in it called latent heat whereas the boiling water does not have this hidden heat.
Question 9. Name A, B, C, D, E and F in the following diagram showing change in its state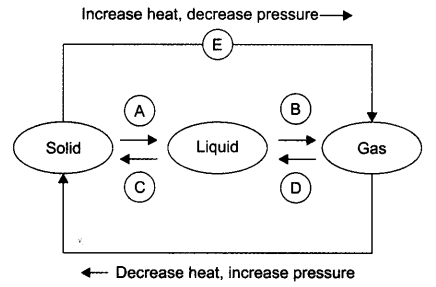
Answer: A —> Liquefication/melting/fusion B —> Vapourisation/evaporation C—>Condensation D—> Solidification E —> Sublimation F —> Sublimation
EXTRA QUESTIONS
1.Define matter. Explain its three states with examples.
- Answer:
- Matter is anything that has mass and occupies space. It exists in three states:
- Solid: Particles are tightly packed and have fixed shape and volume (e.g., ice).
- Liquid: Particles are loosely packed, have a definite volume, but no fixed shape (e.g., water).
- Gas: Particles are far apart, have neither a fixed shape nor volume, and expand to fill the container (e.g., air).
- Matter is anything that has mass and occupies space. It exists in three states:
2. What is the effect of heat on the state of matter? Describe how heating causes a change in state using water as an example.
- Answer:
- Heat causes particles to gain energy and move more rapidly. For example, when water is heated:
- Solid to liquid (melting): Ice melts at 0°C to form liquid water.
- Liquid to gas (evaporation/boiling): Water boils at 100°C to form steam (gas).
- Heat causes particles to gain energy and move more rapidly. For example, when water is heated:
3. Differentiate between solid, liquid, and gas in terms of arrangement of particles, energy, and movement.
- Answer:
- Solid: Particles are closely packed, vibrate in fixed positions, and have low energy.
- Liquid: Particles are loosely packed, can slide over each other, and have more energy than solids.
- Gas: Particles are far apart, move freely in all directions, and have high energy.
4. Explain how the intermolecular forces vary between solids, liquids, and gases.
- Answer:
- Solid: Strong intermolecular forces, particles are held in fixed positions.
- Liquid: Weaker intermolecular forces, particles can move past each other.
- Gas: Very weak intermolecular forces, particles move freely and are far apart.
5. What is meant by “melting point”? How does it vary for different substances?
- Answer:
- The melting point is the temperature at which a solid changes into a liquid. It varies for different substances depending on the strength of the intermolecular forces. For example, ice melts at 0°C, while iron melts at 1538°C.
6. Describe the process of evaporation. What factors affect the rate of evaporation?
- Answer:
- Evaporation is the process where molecules at the surface of a liquid gain enough energy to escape into the air as gas. Factors affecting evaporation include temperature, surface area, and air movement.
7. Explain how the gas laws (Boyle’s law, Charles’ law) relate to the behavior of gases in terms of temperature, pressure, and volume.
- Answer:
- Boyle’s Law: Pressure and volume are inversely related at constant temperature (P ∝ 1/V).
- Charles’ Law: Volume and temperature are directly related at constant pressure (V ∝ T).
8. What is sublimation? Give an example of a substance that undergoes sublimation.
- Answer:
- Sublimation is the process where a solid directly changes into a gas without passing through the liquid state. An example is dry ice (solid CO₂).
9. Explain the difference between boiling and evaporation.
- Answer:
- Boiling: Happens at a specific temperature (boiling point) throughout the liquid; involves heat.
- Evaporation: Occurs at any temperature below the boiling point, only at the surface of the liquid.
10. Why does the temperature of a substance not increase during the phase change from solid to liquid or from liquid to gas, even though heat is being supplied?
- Answer:
- During phase changes, heat is used to overcome the intermolecular forces between particles, which does not increase the temperature. The heat energy is used to change the state rather than to increase the kinetic energy of particles.
11. What is condensation? Give real-life examples where condensation occurs.
- Answer:
- Condensation is the process where gas changes into a liquid when it loses heat. Examples: Water droplets on a cold glass or the formation of clouds.
12. Describe how the states of matter can be converted from one to another with examples.
- Answer:
- Solid to Liquid (Melting): Ice to water.
- Liquid to Gas (Boiling): Water to steam.
- Gas to Liquid (Condensation): Steam to water.
- Liquid to Solid (Freezing): Water to ice.
13. How does the density of a substance change as it changes from solid to liquid or liquid to gas?
- Answer:
- Generally, the density of a substance decreases as it changes from solid to liquid to gas because the particles spread out further in each state. However, water is an exception; its density decreases when it freezes.
14. What is the relationship between kinetic energy of particles and the temperature of a substance?
- Answer:
- The kinetic energy of particles increases with an increase in temperature. As the temperature rises, the particles move faster and have higher kinetic energy.
15. Explain the role of intermolecular forces in determining the physical properties of matter.
- Answer:
- Intermolecular forces determine the state, shape, and behavior of matter. Strong forces result in solids, weak forces result in gases, and liquids have intermediate forces. These forces also affect properties like boiling point, melting point, and viscosity.
16. How can we separate the components of a mixture based on their physical states? Give examples.
- Answer:
- Mixtures can be separated using techniques like:
- Filtration: Separates solids from liquids (e.g., sand and water).
- Evaporation: Separates dissolved solids from liquids (e.g., salt from seawater).
- Distillation: Separates liquids based on boiling points (e.g., separating alcohol from water).
- Mixtures can be separated using techniques like:
17. Define and explain the term “latent heat” in relation to phase changes.
- Answer:
- Latent heat is the heat required to change the state of a substance without changing its temperature. For example, the latent heat of fusion is the heat required to convert a solid to a liquid at its melting point.
18. Why does ice feel colder than water, even though both are at the same temperature?
- Answer:
- Ice feels colder because it absorbs more heat from the skin to melt (latent heat of fusion), whereas water doesn’t need to absorb as much heat to stay at the same temperature.
19. How does the process of diffusion work in gases and liquids? Give examples.
- Answer:
- Diffusion is the movement of particles from an area of higher concentration to an area of lower concentration. In gases, it happens rapidly (e.g., perfume spreading in a room). In liquids, it is slower (e.g., sugar dissolving in water).
20. Explain why gases are highly compressible and solids and liquids are not.
- Answer:
- Gases are highly compressible because their particles are far apart and have a lot of empty space between them. Solids and liquids have particles that are closely packed, leaving little space for compression.
For the official Class 10 Mathematics Solutions, you can visit:
- NCERT Textbooks (for Class 10):
