The chapter Chemical Effects of Current in Class 8 Science (Oxford Book) explores how electric current can cause chemical changes. It introduces key concepts like electrolysis, conductors, and the chemical reactions involved in electric circuits. These solutions are designed to help students understand the topic clearly and answer textbook questions with confidence.
Class 8 Science Chemical Effects of Current Oxford- Textbook
II. Very Short Answer Type Questions
A. Define the following:
- Ions
Charged particles formed when atoms lose or gain electrons. - Anode
The positive electrode where oxidation occurs during electrolysis. - Cathode
The negative electrode where reduction occurs during electrolysis. - Electrolyte
A substance (usually a solution) that conducts electricity by the movement of ions. - Electrolysis
A chemical process in which electrical energy is used to cause a non-spontaneous chemical reaction by passing electric current through an electrolyte.
III. Short Answer Type Questions
- What charged particles make it possible for an electric current to flow through a solution of a salt in water?
Answer: Ions (both positive and negative ions) carry the electric current through the solution. - Name one physical observation by which we can conclude that a chemical reaction takes place during electrorefining.
Answer: The pure metal is deposited on the cathode, which can be seen as a shining layer forming. - Does the presence of salt in water increase or decrease its conductivity?
Answer: Increase — Salt increases the number of ions, so water conducts electricity better. - List two applications of electroplating.
Answer:- To prevent corrosion of metals (e.g., chrome plating on car parts).
- To decorate objects with a shiny layer of precious metals like gold or silver.
- What is electrorefining?
Answer: Electrorefining is the process of purifying a metal by using electrolysis, where impure metal is made the anode, pure metal is deposited on the cathode, and impurities either fall off or remain in the solution.
IV. Long Answer Type Questions
1. Explain why we are more likely to get an electric shock if we operate an electrical appliance with wet hands than with dry hands.
Answer:
Water contains dissolved salts and minerals which make it a good conductor of electricity. When our hands are wet, electricity passes more easily through the water on our skin and into our body, increasing the chance of electric shock. Dry hands have less moisture and are poor conductors, so the risk of shock is lower.
2. With the help of a neat and labelled diagram explain in simple terms how electrolysis takes place. Take the example of sodium chloride solution.
Answer:
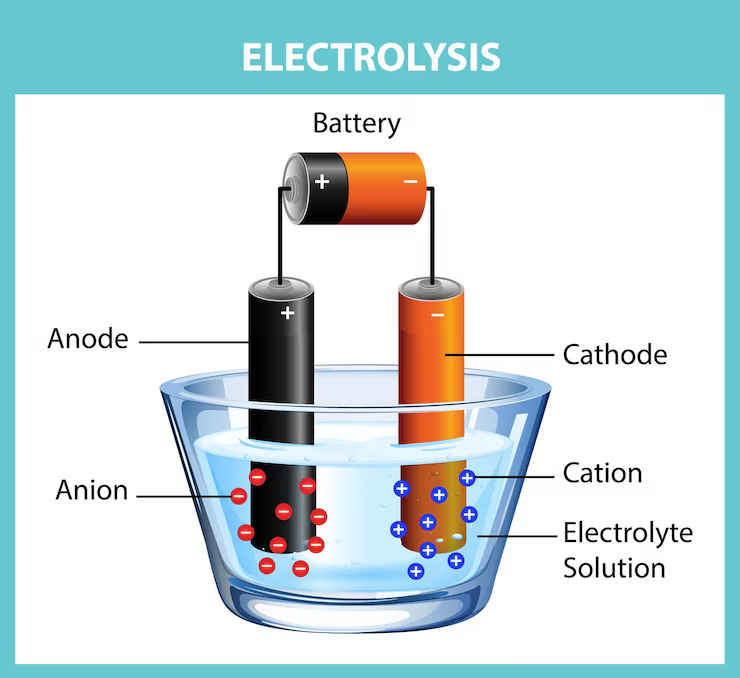
- Electrolysis of sodium chloride solution (brine) involves passing electric current through the solution using two electrodes: anode (positive) and cathode (negative).
- At the anode, chloride ions (Cl⁻) lose electrons (oxidation) and form chlorine gas (Cl₂) which bubbles out.
- At the cathode, hydrogen ions (H⁺) from water gain electrons (reduction) and form hydrogen gas (H₂).
- The solution also contains sodium ions (Na⁺) and hydroxide ions (OH⁻), which remain in the solution and form sodium hydroxide (NaOH).
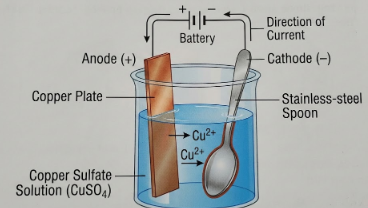
3. What is electroplating? How is it done? What is the purpose of electroplating? Draw a simple labelled diagram to show the electroplating of a stainless-steel spoon with copper.
Answer:
Electroplating is a chemical process where a thin layer of a metal is coated onto another metal object using an electric current.1 .It is an application of the chemical effect of electric current.
The process takes place in an electrolytic cell. Here is how it works, using the example of plating copper onto a spoon:
- The Setup: You need two electrodes (conductors) and an electrolyte (a liquid that conducts electricity).
- The Cathode (Negative Electrode): The object to be plated (e.g., the stainless-steel spoon) is connected to the negative terminal of the battery.
- The Anode (Positive Electrode): A strip of the metal you want to coat with (e.g., a pure copper plate) is connected to the positive terminal.
- The Electrolyte: A salt solution containing the metal to be plated is used (e.g., Copper Sulfate solution, CuSO4).
When electricity is passed through the solution:
- The copper sulfate breaks down into copper ions (Cu^2+) and sulfate ions.
- The positive copper ions are attracted to the negative terminal (the spoon) and get deposited on it, forming a reddish-brown layer.
- At the same time, an equal amount of copper dissolves from the copper plate (anode) into the solution to replace the lost copper ions.
Electroplating is primarily done for two reasons:
- Protection: To protect the underlying metal from corrosion and rusting (e.g., iron bridges are coated with zinc).3
- Appearance: To make dull or inexpensive metals look shiny and attractive (e.g., coating artificial jewelry with gold or silver).4
- Hardening: To make surfaces harder and more resistant to scratching (e.g., chromium plating on car parts).
4. With the help of a diagram, explain the process of electrorefining.
Answer:
Electrorefining is the process of purifying impure metals using electrolysis. It is commonly used to refine metals like copper, zinc, tin, and silver.
Here is the step-by-step process using Copper as an example, as it is the most common textbook case.
The Setup
- Anode (Positive Electrode): A thick block of impure metal (e.g., impure copper) is connected to the positive terminal.
- Cathode (Negative Electrode): A thin strip of pure metal (e.g., pure copper) is connected to the negative terminal.
- Electrolyte: A water-soluble salt solution of the metal to be refined (e.g., Copper Sulfate solution acidified with dilute Sulfuric Acid).
The Process
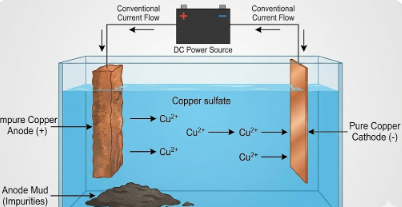
When an electric current is passed through the electrolyte:
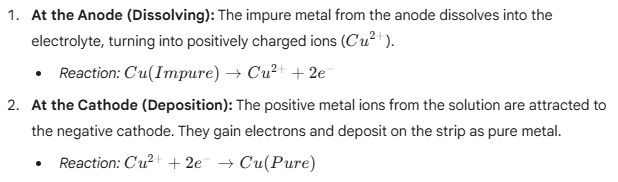
- The Result: Over time, the thick impure anode gets thinner (eaten away), and the thin pure cathode gets thicker as pure metal builds up on it.
What happens to the impurities?
- Soluble impurities dissolve into the solution.
- Insoluble impurities (like gold, silver, platinum) do not dissolve. Instead, they fall to the bottom of the tank below the anode. This sediment is called Anode Mud or Anode Sludge.
Class-wise Solutions
Class 12:
Class 12 Physics – NCERT Solutions
Class 12 Chemistry – NCERT Solutions
Class 11:
- Class 11 Physics – NCERT Solutions
- Class 11 Chemistry – NCERT Solutions
- Class 11 Biology – NCERT Solutions
- Class 11 Math – NCERT Solutions
Class 10:
Class 9:
Class 8: Class 8 Science Chemical Effects of Current Oxford and NCERT Solution -Explore Below
Class 7:
Class 6:
Subject-wise Solutions
Physics:
Chemistry:
Biology:
Math:
- Class 11 Math – NCERT Solutions
- Class 10 Math – NCERT Solutions
- Class 9 Math – NCERT Solutions
- Class 8 Math – NCERT Solutions
Science:
- Class 10 Science – NCERT Solutions
- Class 9 Science – NCERT Solutions
- Class 8 Science – Oxford Solutions
- Class 7 Science – Oxford Solutions
- Class 6 Science – Oxford Solutions
NEET BIOLOGY
- Evolution
- Breathing and Exchange of Gases
- Anatomy of Flowering Plants
- Body Fluids and Circulation
- Human Health and Disease
- Microbes in Human Welfare
- Cell Cycle and Cell Division
- Biotechnology and Its Applications
- Biodiversity and Conservation
- Morphology of Flowering Plants
For the official Mathematics Solutions, you can visit:
- NCERT Textbooks :
he chapter Class 8 Science Chemical Effects of Current Oxford helps students understand how electricity can bring about chemical changes in substances. By learning about electroplating, electrolysis, and the role of conductors and insulators, students gain a deeper understanding of real-life applications of science. The solutions provided for Class 8 Science Chemical Effects of Current Oxford make complex ideas easier to grasp and support effective exam preparation.
With regular practice and revision of Class 8 Science Chemical Effects of Current Oxford, students can strengthen their scientific thinking and perform better in assessments. Keep exploring the concepts in Class 8 Science Chemical Effects of Current Oxford to build a strong foundation in physics and chemistry.
