Atoms and molecules form the basic foundation of Chemistry. Every substance around us – the air we breathe, the water we drink, the food we eat – is made up of tiny building blocks called atoms and molecules. Understanding how these particles combine, interact, and follow certain rules helps us explain chemical reactions and the properties of matter.
In Class 9 Science (Chapter 3: Atoms and Molecules, NCERT/Grade 9), students learn about the historical development of atomic theory, Dalton’s atomic theory, laws of chemical combination, symbols and formulae of elements and compounds, and the concept of mole. These concepts not only build the base for higher classes but also develop a logical approach to Chemistry for both Indian and international learners.

Concept Notes: Class 8 Science – Class 9 Science Ch 3 Atoms and Molecules
1. Introduction
Everything in the universe is made up of matter, and matter is made of extremely small particles. Ancient Indian and Greek philosophers already imagined that matter could be divided into smaller and smaller pieces until a stage is reached where it cannot be divided further. In India, Maharshi Kanad called these particles parmanu, and in Greece, Democritus used the term atomos, meaning indivisible.
Modern science proved that these early ideas were correct in principle. The discovery of atoms, molecules, and laws of chemical combination gave a scientific foundation to chemistry.
2. Laws of Chemical Combination
Chemists observed that elements always combine in simple and definite ways. This gave rise to two important laws:
(a) Law of Conservation of Mass (Lavoisier, 1789)
Mass can neither be created nor destroyed in a chemical reaction. This means the total mass of reactants is always equal to the total mass of products.
Example:
When calcium carbonate decomposes,
CaCO₃ → CaO + CO₂
100 g of CaCO₃ produces 56 g CaO and 44 g CO₂. Mass before = 100 g, after = 100 g.
(b) Law of Constant Proportion (Proust, 1799)
A given compound always contains the same elements in the same fixed proportion by mass, no matter where it is obtained from.
Example:
Water (H₂O) always contains hydrogen and oxygen in the mass ratio of 1:8, whether from rain, river, or laboratory.
3. Dalton’s Atomic Theory (1808)
John Dalton explained these laws by proposing the Atomic Theory. His postulates were:
- All matter is made of tiny, indivisible particles called atoms.
- Atoms of a given element are identical in mass and properties.
- Atoms of different elements are different in mass and properties.
- Atoms cannot be created or destroyed.
- Atoms combine in simple whole-number ratios to form compounds.
- In reactions, atoms only rearrange; they are not destroyed.
👉 Though later discoveries showed that atoms are divisible, Dalton’s theory remains the basis of modern chemistry.
4. Atoms and Their Symbols
An atom is the smallest particle of an element that takes part in a chemical reaction. Atoms are very tiny; their size is measured in nanometres (1 nm = 10⁻⁹ m).
To avoid writing long names of elements, scientists use symbols. J. J. Berzelius introduced the modern system.
- Hydrogen → H
- Oxygen → O
- Carbon → C
- Sodium → Na (Latin: Natrium)
- Potassium → K (Latin: Kalium)
👉 Writing symbols correctly is important. For example, CO = carbon monoxide (a compound), while Co = cobalt (an element).
5. Atomic Mass
Atoms are so small that their mass cannot be measured in grams. Instead, a relative scale is used.
- Earlier, hydrogen was used as a reference, but later carbon-12 isotope was chosen.
- 1 atomic mass unit (amu or u) = 1/12 the mass of a carbon-12 atom.
Approximate atomic masses:
- H = 1 u, C = 12 u, O = 16 u, Na = 23 u, Cl = 35.5 u.
6. Molecules
Atoms do not usually exist alone. They combine to form molecules, which are the smallest particles of a substance having its properties.
Types:
- Molecules of elements – contain atoms of the same element.
- H₂, O₂, N₂, Cl₂, S₈, P₄
- Molecules of compounds – contain atoms of different elements.
- H₂O, CO₂, NH₃, CH₄
7. Ions and Radicals
Atoms or groups of atoms can carry charge → these are called ions.
- Cations: positively charged (Na⁺, Mg²⁺, Al³⁺).
- Anions: negatively charged (Cl⁻, O²⁻, SO₄²⁻, NO₃⁻).
Some common polyatomic radicals with valency:
- Hydroxide (OH⁻, valency 1)
- Nitrate (NO₃⁻, valency 1)
- Carbonate (CO₃²⁻, valency 2)
- Sulphate (SO₄²⁻, valency 2)
- Ammonium (NH₄⁺, valency 1)
8. Valency
Valency is the combining capacity of an atom. It depends on the number of electrons lost, gained, or shared to complete the outer shell.
Examples:
- H = 1, O = 2, N = 3, C = 4
- Na = 1, Mg = 2, Al = 3, Cl = 1
👉 Valency is always a whole number and is the basis of writing formulae.
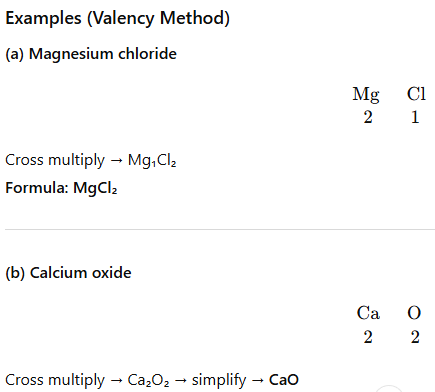
9. Writing Chemical Formulae (Valency Method)
Rules:
- Write symbols of elements/ions.
- Write valencies below them.
- Cross–multiply valencies.
- Simplify if needed.
- Use brackets for polyatomic ions if more than one.
Examples:
- Magnesium chloride: Mg (2), Cl (1) → MgCl₂
- Calcium oxide: Ca (2), O (2) → CaO
- Aluminium chloride: Al (3), Cl (1) → AlCl₃
- Copper nitrate: Cu (2), NO₃ (1) → Cu(NO₃)₂
- Calcium carbonate: Ca (2), CO₃ (2) → CaCO₃
10. Molecular Mass and Formula Unit Mass
- Molecular mass = sum of atomic masses of atoms in a molecule.
- H₂O = 2 × 1 + 16 = 18 u
- CO₂ = 12 + 32 = 44 u
- Formula unit mass = used for ionic compounds (like NaCl).
- NaCl = 23 + 35.5 = 58.5 u
11. Importance of Formulae
- They tell us the exact composition of compounds.
- They help in writing chemical equations.
- They are essential for calculating molecular mass and understanding reactions.
12. Summary
- All matter is made up of atoms and molecules.
- Laws of conservation of mass and constant proportion explain how substances combine.
- Dalton’s Atomic Theory gave the first scientific explanation.
- Atoms are represented by symbols; their mass is measured in atomic mass units.
- Molecules may be of elements or compounds.
- Ions and radicals are charged species that also form compounds.
- Valency is the basis of chemical formula writing.
- Formulae allow us to calculate molecular and formula unit masses.
Class 9 Science Ch 3 Atoms and Molecules – Textbook Solution
Intext Question Page 27
1. In a reaction 5.3 g of sodium carbonate reacted with 6 g of ethanoic acid. The products were 2.2 g of carbon dioxide, 0.9 g water and 8.2 g of sodium ethanoate. Show that these observations are in agreement with the law of conservation of mass carbonate.
Answer.
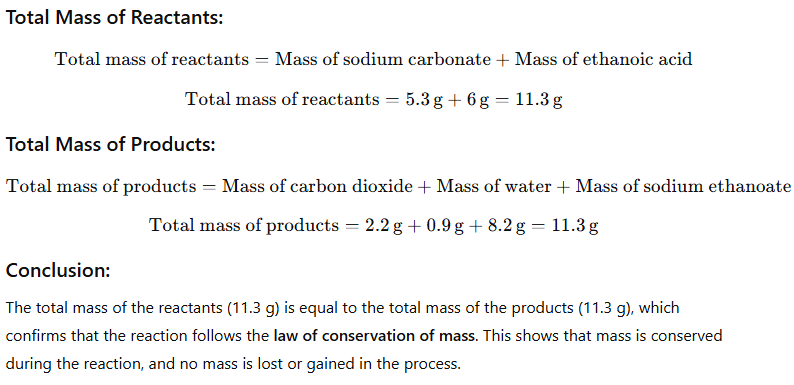
Question 2. Hydrogen and oxygen combine in the ratio of 1 : 8 by mass to form water. What mass of oxygen gas would be required to react completely with 3 g of hydrogen gas?
Answer: Hydrogen and oxygen combine in the ratio 1 : 8 by mass.
For 1 g hydrogen, oxygen required = 8 g.
For 3 g hydrogen, oxygen required = 3 × 8 = 24 g.
Therefore, 24 g of oxygen gas is required.
Question 3. Which postulate of Dalton’s atomic theory is the result of the law of conservation of mass?
Answer: The postulate of Dalton’s atomic theory which explains the law of conservation of mass is:
“Atoms can neither be created nor destroyed in a chemical reaction. They only rearrange to form new substances.”
Question 4. Which postulate of Dalton’s atomic theory can explain the law of definite proportions?
Answer: The postulate of Dalton’s atomic theory which explains the law of definite proportions is:
“Atoms of a given element have identical mass and chemical properties, and atoms combine in simple whole number ratios to form compounds.”
Intext Question Page 27
Question 1. Write down the formulae of
(i) Sodium oxide
(ii) Aluminium chloride
(iii) Sodium sulphide
(iv) Magnesium hydroxide
Answer:
(i) Sodium oxide – Na₂O
(ii) Aluminium chloride – AlCl₃
(iii) Sodium sulphide – Na₂S
(iv) Magnesium hydroxide – Mg(OH)₂
Question 2. What is meant by the term chemical formula?
Answer: The chemical formula of the compound is a symbolic representation of its composition, e.g., chemical formula of sodium chloride is NaCl.
Question 3. How many atoms are present in a
(i) H2S molecule and
(ii) P043- ion?
Answer: (i) H2S —> 3 atoms are present
(ii) P043- —> 5 atoms are present
Intext Questions – Page 35
Question 1. Calculate the molecular masses of H2, O2, Cl2, C02, CH4, C2H2,NH3, CH3OH.
Answer: The molecular masses are:
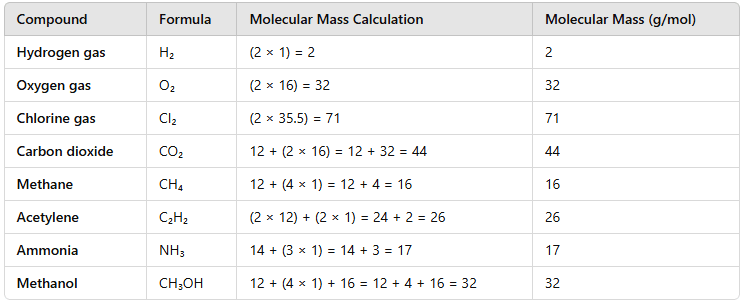
Question 2.Calculate the formula unit masses of ZnO, Na2O, K2C03, given atomic masses of Zn = 65 u, Na = 23 u, K = 39 u, C = 12 u, and O = 16 u.
Answer: The formula unit mass of
(i) ZnO = 65 u + 16 u = 81 u
(ii) Na2O = (23 u x 2) + 16 u = 46 u + 16 u = 62 u
(iii) K2C03 = (39 u x 2) + 12 u + 16 u x 3
= 78 u + 12 u + 48 u = 138 u
Questions From NCERT Textbook for Class 9 Science
Question 1. A 0.24 g sample of compound of oxygen and boron was found by analysis to contain 0.096 g of boron and 0.144 g of oxygen. Calculate the percentage composition of the compound by weight.
Answer: Boron and oxygen compound —> Boron + Oxygen
0.24 g —> 0.096 g + 0.144 g
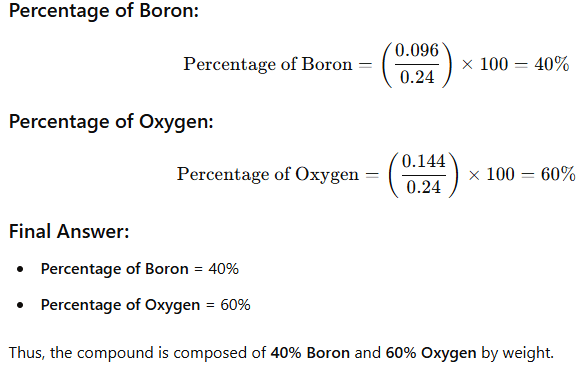
Question 2. When 3.0 g of carbon is burnt in 8.00 g oxygen, 11.00 g of carbon dioxide is produced. What mass of carbon dioxide will be formed when 3.00 g of carbon is burnt in 50.00 g of oxygen? Which law of chemical combination will govern your answer?
Answer: When 3.0 g carbon reacts with 8.0 g oxygen, it forms 11.0 g carbon dioxide.
So, the ratio of carbon : oxygen = 3 : 8.
If 3.0 g carbon is burnt in 50.0 g oxygen, still only 8.0 g oxygen will combine (because carbon is limiting).
Therefore, carbon dioxide formed = 3.0 g + 8.0 g = 11.0 g.
The law that governs this is the Law of Constant Proportions (a compound always contains the same elements in the same fixed ratio by mass).
Question 3. What are poly atomic ions? Give examples.
Answer: The ions which contain more than one atoms (same kind or may be of different kind) and behave as a single unit are called polyatomic ions e.g., OH–, SO42-, CO32-.
Question 4. Write the chemical formulae of the following:
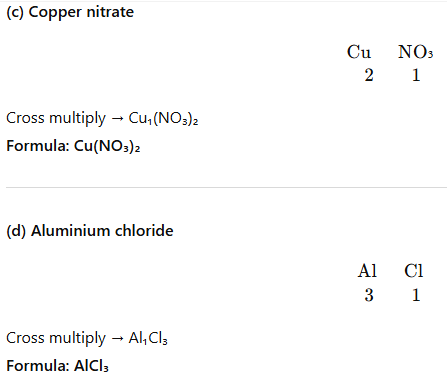
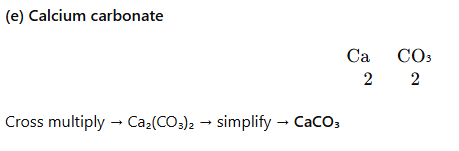
Question 5. Give the names of the elements present in the following compounds:
(a) Quick lime
(b) Hydrogen bromide
(c) Baking powder
(d) Potassium sulphate.
Answer: (a) Quick lime —> Calcium oxide
Elements —> Calcium and oxygen
(b) Hydrogen bromide
Elements —> Hydrogen and bromine
(c) Baking powder —> Sodium hydrogen carbonate
Elements —> Sodium, hydrogen, carbon and oxygen
(d) Potassium sulphate
Elements —> Potassium, sulphur and oxygen
Question 6. Calculate the molar mass of the following substances.
(a) Ethyne, C2H2
(b) Sulphur molecule, S8
(c) Phosphorus molecule, P4 (Atomic mass of phosphorus = 31)
(d) Hydrochloric acid, HCl
(e) Nitric acid, HNO3
Answer: The molar mass of the following: [Unit is ‘g’]
(a) Ethyne, C2H2 = 2 x 12 + 2 x 1 = 24 + 2 = 26 g
(b) Sulphur molecule, S8 = 8 x 32 = 256 g
(c) Phosphorus molecule, P4=4 x 31 = i24g
(d) Hydrochloric acid, HCl = 1 x 1 + 1 x 35.5 = 1 + 35.5 = 36.5 g
(e) Nitric acid, HN03 = 1 x 1 + 1 x 14 + 3 x 16 = 1 + 14 + 48 = 63 g
Worksheet – Class 9 Science Ch 3 Atoms and Molecules
Section A – Fill in the blanks
- The law of conservation of mass was given by __________.
- The law of constant proportion was given by __________.
- The smallest particle of an element that takes part in a chemical reaction is called __________.
- The symbol of sodium is __________ and of potassium is __________.
- The atomic mass of oxygen is __________ u.
Section B – True or False
- Atoms can be created and destroyed in chemical reactions. ( )
- Water always has hydrogen and oxygen in the ratio 1:8 by mass. ( )
- Cations are negatively charged ions. ( )
- H₂, O₂ and N₂ are molecules of elements. ( )
- CaCO₃ is the chemical formula of calcium carbonate. ( )
Section C – Match the following
- Na → (a) Oxygen
- O → (b) Potassium
- K → (c) Sodium
- Fe → (d) Iron
- Cl → (e) Chlorine
Section D – Short Answer Questions
- State the law of conservation of mass with an example.
- State the law of constant proportion with an example.
- Write any three postulates of Dalton’s Atomic Theory.
- Define: (a) Atomic mass (b) Molecular mass.
- Differentiate between molecule of an element and molecule of a compound with examples.
Section E – Formula Writing (Valency Method)
Write the chemical formulae of:
- Sodium oxide
- Aluminium chloride
- Sodium sulphide
- Magnesium hydroxide
- Calcium carbonate
- Copper nitrate
- Potassium sulphate
- Ammonium chloride
Section F – Higher Thinking Questions
- Why do we use atomic mass unit instead of gram for atoms?
- Why do we use brackets while writing formulae of compounds containing polyatomic ions?
- Which two laws of chemical combination can be explained by Dalton’s Atomic Theory?
- Why does Na combine with Cl in 1:1 ratio while Mg combines with Cl in 1:2 ratio?
- Why is it important to know the valency of an element?
Here’s your PDF version of the worksheet with introduction, questions, and answer key:
📂 Download: Atoms_and_Molecules_Worksheet_with_Answers.pdf
You can access the official NCERT Solutions for Class 10 Mathematics on the NCERT website at the following link:
NCERT Class 10 Mathematics Solutions
This page will guide you to the textbook and solutions, as provided by the National Council of Educational Research and Training (NCERT).
